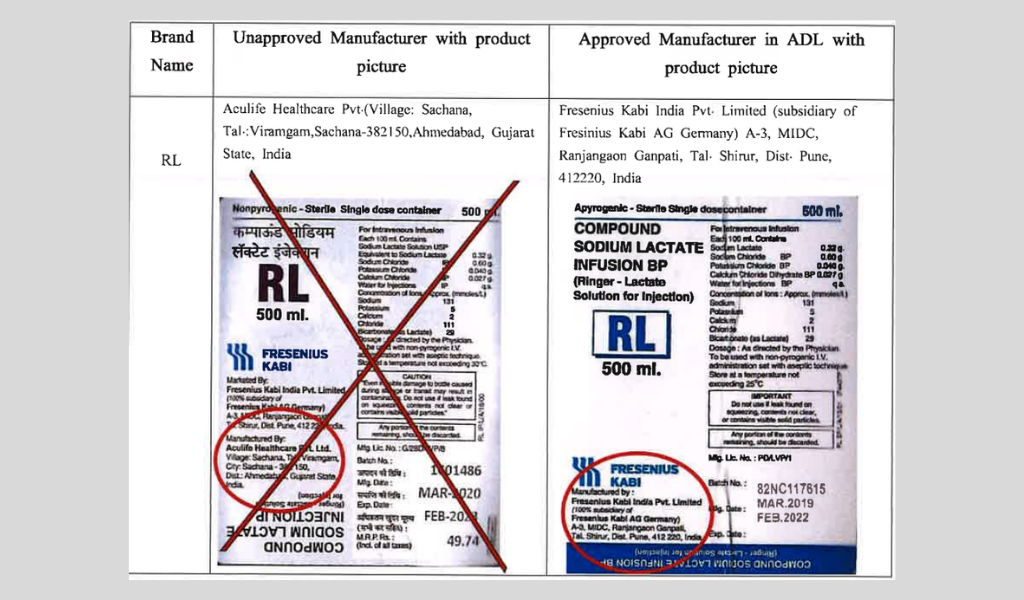
F&D Authority Bans Use Of False IV Brand
The IV Brand RL, imported and being used in the market has been found to have quality issues as per an announcement made by Maldives Food & Drug Authority. Hence, the authority warns people to stop the use of the product effective immediately.
Food & Drug Authority has also stressed on the fact that the authority had not given approval for the RV brand IV products to be imported and be sold in the country by the company selling it. In addition, the authority highly requests the public to report any medical facilities, clinics, hospitals and pharmacies that are found selling the said product.
Please contact 7200321 for complaints and reports.